Genetic ancestry associated with molecular subtypes, prognosis of childhood ALL
Apr 18, 2022
4 minutes reading
Source/Disclosures Published by:
disclosures:
Yang reports grants from NIH during the conduct of the research and from NIH outside of the submitted work, as well as a patent pending for methods for determining the benefit of chemotherapy. See the study for the relevant financial disclosures from all other authors. Rabin reports no relevant financial disclosures.
ADD TOPIC TO EMAIL ALERTS
Receive an email when new articles are posted
Enter your e-mail address to receive an e-mail when there are new articles on . ” data-action = “subscribe” > Subscribe
We were unable to process your request. Try again later. If this problem persists, please contact customerservice@slackinc.com.
Back to Healio
Molecular subtypes and prognosis of acute lymphoblastic leukemia appeared to be associated with genetic ancestry, suggesting a possible genetic basis for racial and ethnic disparities in ALL, according to a study in JAMA Oncology.
“It is very clear that ALL childhood biology differs by genetic ancestry and the biology of this childhood cancer has great global diversity,” Shawn HR Lee, MD† postdoctoral researcher, and Jun J. Yang, PhD† vice chair in the division of pharmacy and pharmaceutical sciences, both at St. Jude Children’s Research Hospital, Healio said in a joint statement.
Data derived from Lee SHR, et al. JAMA Oncol. 2022;doi:10.1001/jamaoncol.2021.6826.
“In the past, we have always extrapolated treatment protocols developed for white children (children of European descent) to other populations,” they continued. “In the age of personalized medicine, we need to develop biologically guided treatment protocols that also take this racial diversity into account and no longer assume that what works for white children also works for children from other populations.”
Methodology
The analysis included 2,428 children and adolescents with ALL (mean age 7.8 years; 57.8% male; 70.2% ages 1 to 9 years) from the US, Southeast Asia (Singapore and Malaysia) and Latin America (Guatemala) who participated in the frontline clinical trials between March 1, 2000 and November 20, 2020.
Shawn HR Lee
Lee, Yang and colleagues performed RNA sequencing to comprehensively categorize molecular subtypes of ALL and genetic ancestors. They then evaluated genetic ancestry associations with ALL molecular subtypes and treatment outcomes.
The researchers sought to characterize the diversity of ALL biology (i.e., molecular subtypes) in different world populations and determine whether genetic ancestry influences outcome, even with modern therapy.
Key findings
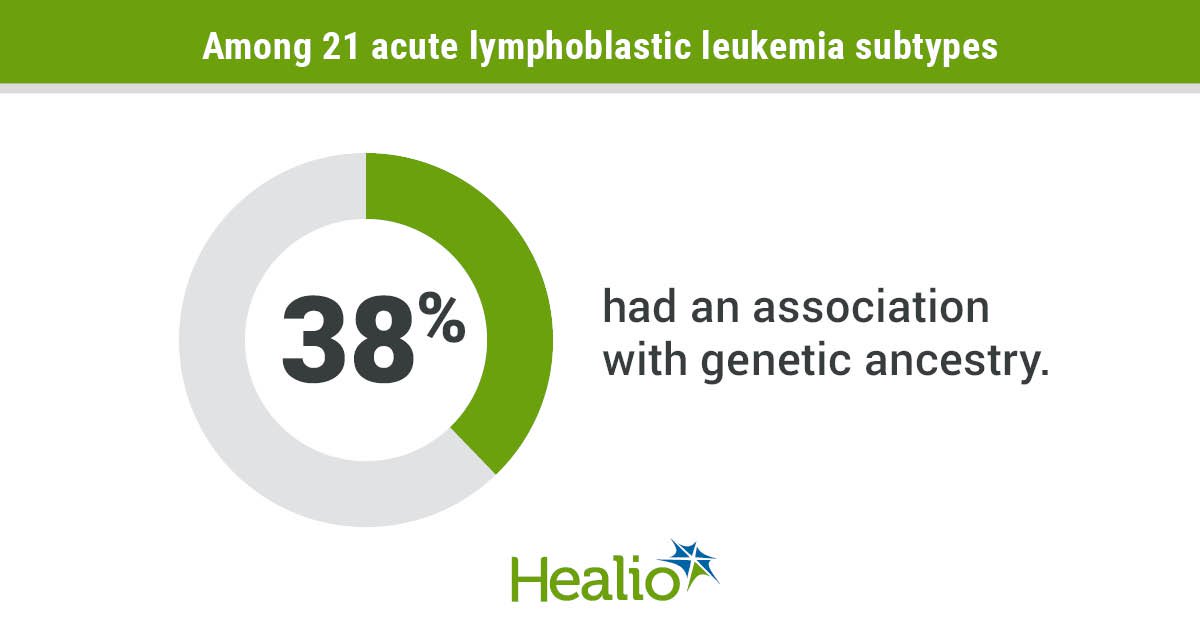
The results showed that eight of the 21 identified ALL subtypes were associated with ancestors.
Within the individual populations, researchers reported:
East Asian ancestry had a positive association with somatic DUX4 (OR = 1.3; 95% CI, 1.16-1.45) and ZNF384 (OR = 1.4; 95% CI, 1.18-1.66 ) gene rearrangements and a negative association with BCR-ABL1-like ALL (OR = 0.79; 95% CI, 0.66-0.92) and T-cell ALL (OR = 0.8; 95% CI, 0, 71-0.9).
Native American ancestry had a positive association with CRLF2 arrangements (OR = 1.48; 95% CI, 1.29-1.69). ETV6-RUNX1 fusion became less common as the percentage of Native American ancestry increased (OR = 0.8; 95% CI 0.7-0.91), with the opposite trend observed for ETV6-RUNX1-like ALL.
Children of African descent (OR = 1.22; 95% CI, 1.07-1.37) had a clear predominance of T-cell ALL compared to children with a high percentage of Native American descent (OR = 0.53; 95% CI, 0.4-0.67).
African ancestry had a positive association with TCF3-PBX1 (OR = 1.49; 95% CI, 1.25-1.76) and a negative association with DUX4 rearrangements (OR = 0.7; 95% CI, 0, 48-0.93; P = 0.01) and hyperdiploidy (OR = 0.77; 95% CI, 0.68-0.86).
As continuous variables, for every 25% increase in ancestry, African and Native American ancestry had an association with worse EFS (African ancestry: HR = 1.2; 95% CI, 1.1-1.4; Native American ancestry: HR = 1.3 95% CI, 1-1.6) and OS (African descent: HR = 1.2; 95% CI, 1.1-1.5; Native American descent, HR, 1.4; 95% CI, 1-1, 8).
Native American and African ancestry remained associated with poor prognosis, even after adjusting for biological subtypes and clinical features, the researchers wrote.
Jun J. Yang
“While we expected some differences in ALL biology due to genetics, we probably didn’t expect to see such obvious differences between some of these new subtypes (for example, DUX4),” Lee and Yang told Healio.
Implications
The results may have simply scratched the surface about the association of genetic ancestry with ALL outcomes, the researchers say.
“Our analyzes were on the conservative side and may not have been powerful enough to sufficiently detect differences for the very rare subtypes; therefore, these differences in biology may be much more extensive than we know,” Lee and Yang told Healio.
They said the next steps would be to evaluate genetic ancestry among larger, global ALL cohorts, particularly in Asian and Middle Eastern countries.
“Overall, we would like to see racial diversity taken center stage as a consideration in both clinical research and patient treatment, with the goal of eliminating racial gaps in survival,” they said.
In an editorial accompanying the study, Karen R. Rabin, MD, PhD† director of the leukemia program at Texas Children’s Hospital and associate professor of pediatrics in the hematology-oncology section at Baylor College of Medicine, wrote that the study “lays a strong foundation for further research,” but highlighted some areas lacking in the Research.
“While this study includes a commendable array of international studies and an impressive total number of participants, it still faces power constraints due to multiple comparisons between the many ALL subtypes and racial and ethnic categories, several of which have relatively small numbers,” Rabin says. . wrote. “Another question requiring further research is how age may interact with genetic ancestry. The frequencies of ALL subtypes vary significantly with age, as do patient outcomes. The current study included only a small number of patients aged 18 to 30.”
References†
Lee SHR, et al. JAMA Oncol. 2022;doi:10.1001/jamaoncol.2021.6826
Rabin KR. JAMA Oncol. 2022;doi:10.1001/jamaoncol.2021.6785.
For more information:
Shawn HR Lee, MD† and June J. Yang, PhD, can be reached at the Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105; email Lee: shawn.lee@stjude.org; email Yang: jun.yang@stjude.org.
ADD TOPIC TO EMAIL ALERTS
Receive an email when new articles are posted
Enter your e-mail address to receive an e-mail when there are new articles on . ” data-action = “subscribe” > Subscribe
We were unable to process your request. Try again later. If this problem persists, please contact customerservice@slackinc.com.
Back to Healio

Comments are closed.