RACE Act led to more required pediatric studies of cancer drugs
Apr 11, 2022
3 minutes reading
Source/Disclosures Published by:
Source:
McKelvey BA, et al. Poster 13. Presented at: American Association for Cancer Research Annual Meeting (hybrid meeting); Apr 8-13, 2022.
disclosures:
Friends of Cancer Research funded this research. McKelvey does not report any relevant financial disclosures. See the abstract for the relevant financial statements of all researchers. Weiner reports being co-founder of Jounce Therapeutics; advisory board roles for BioXcel Therapeutics, Celldex Pharmaceuticals, Celularity, Inc., Cytomx Therapeutics, Immunome, Inc., Jounce Therapeutics, Samyang Pharmaceuticals, and Tessa Therapeutics; and research collaborations with BioXcel Therapeutics.
ADD TOPIC TO EMAIL ALERTS
Receive an email when new articles are posted
Enter your e-mail address to receive an e-mail when there are new articles on . ” data-action = “subscribe” > Subscribe
We were unable to process your request. Try again later. If this problem persists, please contact customerservice@slackinc.com.
Back to Healio
The Research to Accelerate Cures and Equity Act led to an increase in the number of required pediatric trials for recently approved cancer therapies within the first year of the law’s implementation, according to the study findings.
Researchers presented the findings at the annual meeting of the American Association for Cancer Research.
Data derived from McKelvey BA, et al. Poster 13. Presented at: American Association for Cancer Research Annual Meeting (hybrid meeting); Apr 8-13, 2022.
Rationale and Methods
“Significant progress has been made in cancer care and treatment, with many new therapies approved recently. However, many of these exciting developments have not easily translated into new treatment options and approvals for pediatric oncology patients,” Brittany Avin McKelvey, PhD, science policy analyst at Friends of Cancer Research, at Healio. “Many legislative efforts have been made to encourage the study of childhood cancer therapies, including the 2017 passage of the Research to Accelerate Cures and Equity [RACE] Act, which was implemented in August 2020. It is important to assess the impact of the RACE Act on requirements for pediatric studies and drug development for pediatric oncology, as well as to identify additional policy options to maximize its impact.”
To do this, McKelvey and colleagues evaluated all new FDA-approved oncology drug applications or biological license applications between 2019 and 2021, stratifying qualifying drugs by applications approved 1 year before and 1 year after RACE implementation.
Key findings
Researchers identified 19 therapies within the study period, of which 63.2% received approval before the implementation of the RACE Act and 36.8% after it.
The results showed that only 11.8% of approved therapies had indications for pediatric use at the time of first drug administration. Among them, 78.9% of the approved applications received orphan drug designation.
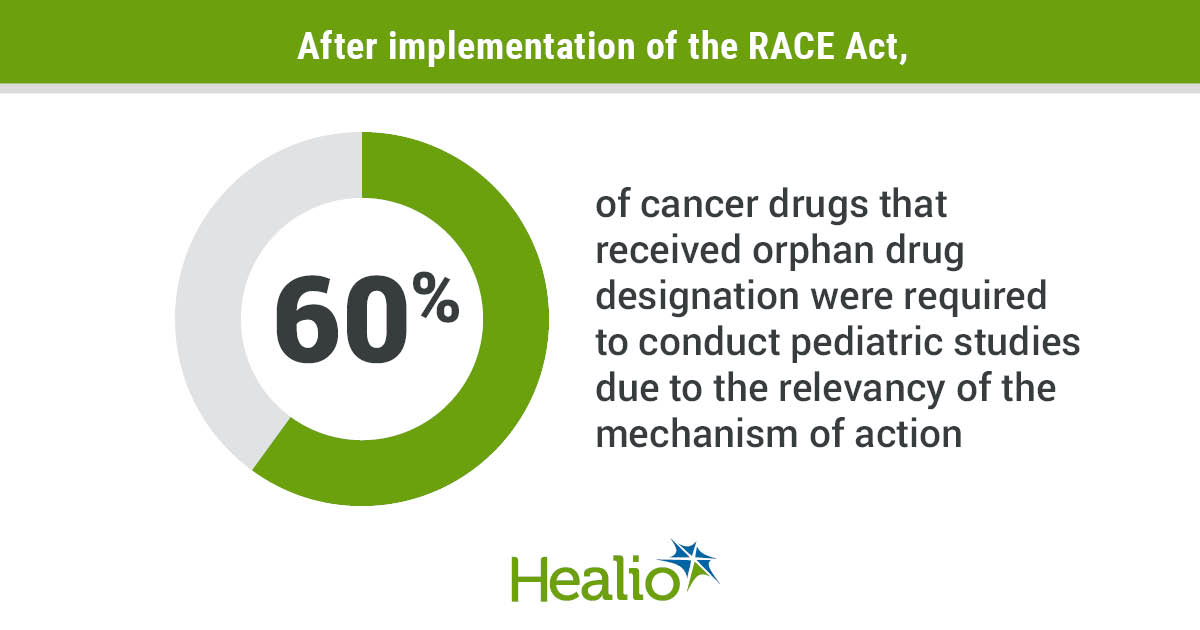
Before the enactment of the RACE Act, 91.7% of approved therapies had a relevant mechanism of action that, according to the researchers, could have required pediatric investigation if the drug application had been submitted after the law’s implementation. Following implementation of the law, 71.4% of cancer therapies received orphan drug designation, of which 60% required a pediatric assessment due to the relevance of the mechanism of action.
Before the enactment of the RACE Act, pediatric clinical trials were not allowed for all therapies, while after the enactment of the Act, 42.9% of the approved cancer therapies required pediatric research.
Brittany Avin McKelvey
“We found a significant increase in the number of required pediatric studies for new oncology therapies following implementation of the RACE Act, with the greatest effect seen in orphan drugs that are no longer automatically exempt,” McKelvey said. “However, many pediatric trial requirements are still waived for therapies with a relevant mechanism of action for pediatric cancers, highlighting the need for additional capabilities to facilitate and encourage robust pediatric trials.”
Implications
Trends are emerging about the effectiveness of the RACE Act in increasing pediatric research on oncology therapies, which could eventually translate into new life-saving treatment options for children with cancer, McKelvey said.
“All required pediatric investigations were postponed,” she said. “Many of these studies require completion of data over a long period of time, for example in 2028. Therefore, we have many years before we can see if the required pediatric studies lead to actionable data that can support labeling changes and incorporation of information to determine appropriate use in pediatric patients.”
The researchers plan to extend their initial analysis beyond the first year of implementation of the RACE Act.
“We were only able to evaluate the requirements of pediatric research and we cannot currently assess whether the studies have been completed and whether they lead to improved pediatric labeling,” McKelvey said. “We hope that by collecting additional data, we can continue to assess the impact of current policies and assess additional opportunities to facilitate and encourage pediatric research.”
Although the pediatric study of cancer drugs has increased, many requirements are still being waived for therapies with a relevant mechanism of action, McKelvey said.
†[That is] because studies are considered ‘impossible or impractical’ due to the prevalence of the mutation or cancer type in the pediatric population,” she added. “Due to the rare prevalence of pediatric cancers and the further wiping out of patients by specific changes, the issue of prevalence is not uncommon.”
According to McKelvey, rare target patients may need innovative study designs, such as platform studies or adaptive study designs, and collaboration to make the impossible or impractical studies possible.
“Further, there is an opportunity to use the data and experience from the adult clinical trials to inform and accelerate pediatric drug development by extrapolating certain safety and efficacy data,” she said. “There may also be an opportunity to expand the eligibility criteria to allow for the enrollment of adolescents in the premarket pivotal pivotal trial to more quickly provide important information about safety and efficacy in this population.”
Ongoing research is essential, according to moderator Louis M. Weiner, MD, director of the Georgetown Lombardi Comprehensive Cancer Center.
“Initial results show that the RACE law has a desired effect, making it important for these drugs to be evaluated in pediatric populations, but it’s a long time frame,” he said. “The assumption is that having more early-stage access will ultimately be beneficial, but it’s important to study the actual impact.”
Perspective
Back upstairs
Richard Gorlick, MD
There has been a huge improvement in the survival rates of children with the most common childhood cancers through optimization of chemotherapy drugs developed in the 1950s through the 1980s.
With the exception of some notable oncology drugs whose early development was through pediatrics – such as asparaginase erwinia chrysanthemi (Rylaze, Jazz Pharmaceuticals), blinatumomab (Blincyto, Amgen), clofarabine, dinutuximab (Unituxin, United Therapeutics), nelarabine (Arranon, Novartis), and tisagenlecleucel (Kymriah, Novartis) – the challenge was the delay or non-performance of studies in the first children. While many cancers in adults do not have a clear counterpart in children, many do. The overlap in the occurrence of different cancers in adult and pediatric populations was only increased by a better molecular understanding of these entities, allowing for greater sophistication in what is recognized as similar or different.
This analysis makes it clearer that some therapies that are beneficial for adults with cancer are likely to offer a similar benefit when tested in children. McKelvey and colleagues evaluated the effectiveness of the RACE Act by determining the number of agents required for pediatric studies by the FDA after implementation compared to earlier time points. They found an increase in the number of drugs requiring pediatric studies following the implementation of the RACE Act – especially for those with orphan drug designations – indicating the effectiveness of the legislation, and further increases are possible through additional mandates.
It is hoped that the RACE Act, by enabling the testing of new drugs in children with cancer, will support further progress in this all-important area.
Richard Gorlick, MD
The University of Texas MD Anderson Cancer Center
disclosures: Gorlick reports no relevant financial disclosures.
ADD TOPIC TO EMAIL ALERTS
Receive an email when new articles are posted
Enter your e-mail address to receive an e-mail when there are new articles on . ” data-action = “subscribe” > Subscribe
We were unable to process your request. Try again later. If this problem persists, please contact customerservice@slackinc.com.
Back to Healio

Comments are closed.